A Modular Fluorescent Probe for Viscosity and PolaritySensing in DNA Hybrid Mesostructures
- Andreas Walther
- Mar 3, 2021
- 1 min read
Read the full article here: Adv. Sci. 2021, 2003740
by S. Ludwanowski, A. Samanta, S. Loescher, C. Barner-Kowollik, and Andreas Walther
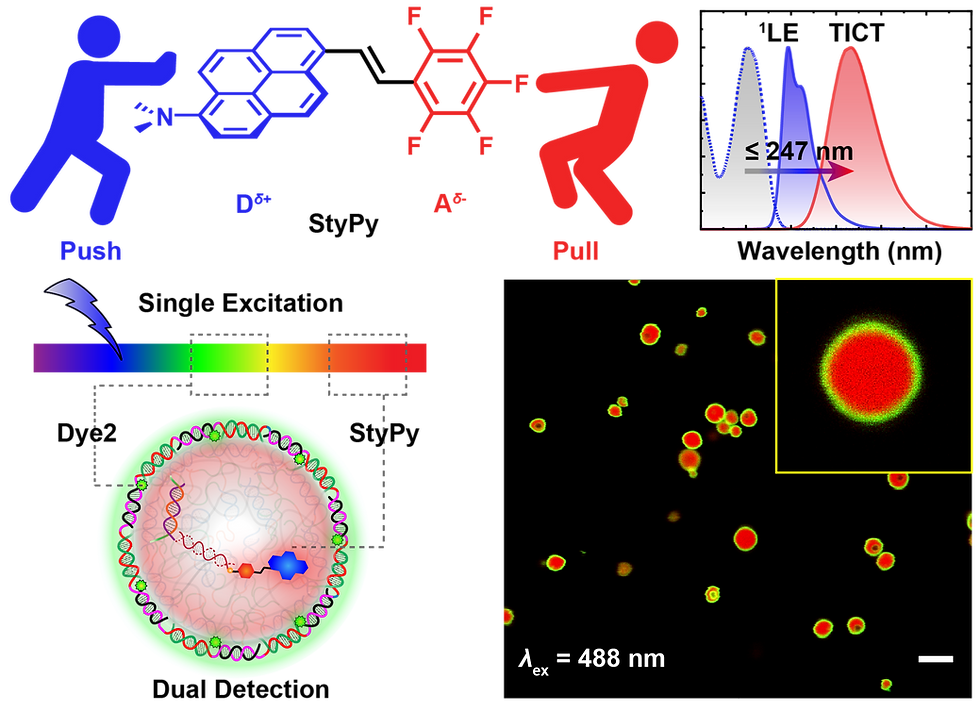
There exists a critical need in biomedical molecular imaging and diagnostics for molecular sensors that report on slight changes to their local microenvironment with high spatial fidelity. Herein, a modular fluorescent probe, termed StyPy, is rationally designed which features i) an enormous and tunable Stokes shift based on twisted intramolecular charge transfer (TICT) processes with no overlap, a broad emission in the far‐red/near‐infrared (NIR) region of light and extraordinary quantum yields of fluorescence, ii) a modular applicability via facile para‐fluoro‐thiol reaction (PFTR), and iii) a polarity‐ and viscosity‐dependent emission. This renders StyPy as a particularly promising molecular sensor. Based on the thorough characterization on the molecular level, StyPy reports on the viscosity change in all‐DNA microspheres and indicates the hydrophilic and hydrophobic compartments of hybrid DNA‐based mesostructures consisting of latex beads embedded in DNA microspheres. Moreover, the enormous Stokes shift of StyPy enables one to detect multiple fluorophores, while using only a single laser line for excitation in DNA protocells. The authors anticipate that the presented results for multiplexing information are of direct importance for advanced imaging in complex soft matter and biological systems.



Yorumlar